Therapeutic Use License
If you are considering solutions/technologies for medical applications for use in regenerative medicine, cell therapy and transplantation, human and animal treatment, disease prevention and diagnosis, please see here.
Standard Financial Terms
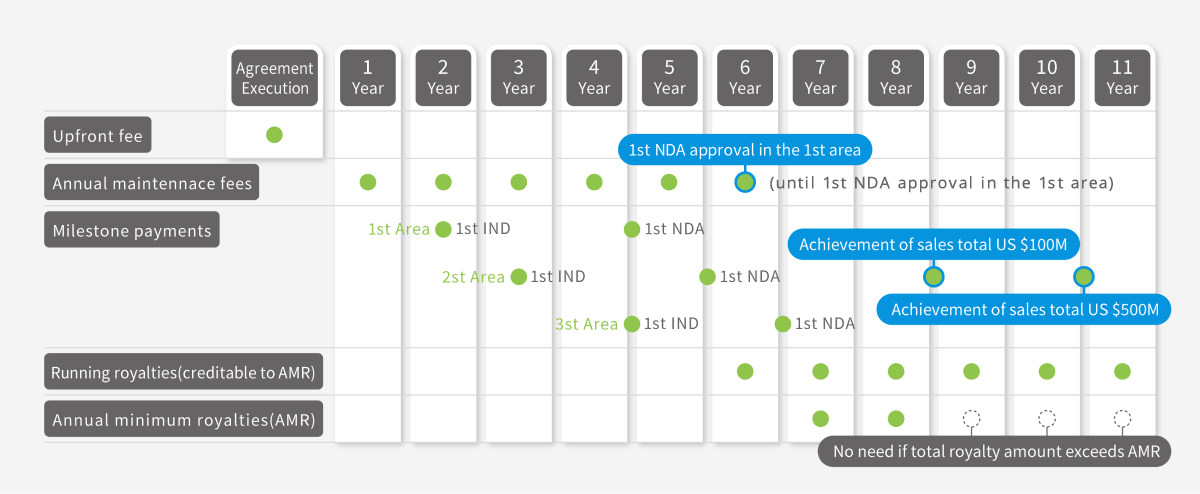
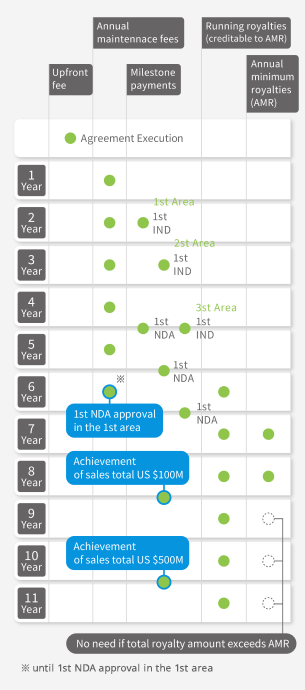
Non-Exclusive worldwide license for each differentiated cell types
(Patents to be licensed: AJ001, AJ002, AJ005, AJ006, AJ018 and AJ066)
(Patents to be licensed: AJ001, AJ002, AJ005, AJ006, AJ018 and AJ066)
| Fees and Royalties | SSE *1 | Non-SSE |
|---|---|---|
| Upfront fee | US$13,000 | US$60,000 |
| Annual maintenance fee (until NDA approval) |
US$12,000 | US$25,000 |
| Milestone payments on first IND applications in each of 3 areas *2 | (in total of 3 areas) US$41,000 |
(in total of 3 areas) US$130,000 |
| Milestone payments on first NDA applications in each of 3 areas *2 |
(in total of 3 areas) US$250,000 |
(in total of 3 areas) US$295,000 |
| Milestone payment on achievement of sales total US$100M |
US$400,000 | |
| Milestone payment on achievement of sales total US$500M |
US$700,000 | |
| Running royalties | 1.5% of sales of final products | |
| Annual minimum royalties (after NDA approval) |
US$20,000 | US$25,000 |
| Fees and Royalties | SSE *1 |
|---|---|
| Upfront fee | US$13,000 |
| Annual maintenance fee (until NDA approval) |
US$12,000 |
| Milestone payments on first IND applications in each of 3 areas *2 | (in total of 3 areas) US$41,000 |
| Milestone payments on first NDA applications in each of 3 areas *2 |
(in total of 3 areas) US$250,000 |
| Milestone payment on achievement of sales total US$100M |
US$400,000 |
| Milestone payment on achievement of sales total US$500M |
US$700,000 |
| Running royalties | 1.5% of sales of final products |
| Annual minimum royalties (after NDA approval) |
US$20,000 |
| Fees and Royalties | Non-SSE |
|---|---|
| Upfront fee | US$60,000 |
| Annual maintenance fee (until NDA approval) |
US$25,000 |
| Milestone payments on first IND applications in each of 3 areas *2 | (in total of 3 areas) US$130,000 |
| Milestone payments on first NDA applications in each of 3 areas *2 |
(in total of 3 areas) US$295,000 |
| Milestone payment on achievement of sales total US$100M |
US$400,000 |
| Milestone payment on achievement of sales total US$500M |
US$700,000 |
| Running royalties | 1.5% of sales of final products |
| Annual minimum royalties (after NDA approval) |
US$25,000 |
∗1 A Small and Startup Entity (SSE) means an entity which (i) has been within 10 years from its foundation, (ii) employs 50 or fewer people, and (iii) has received US$20M or less in total as financial resources.
∗2 3 areas mean North America, Europe and the rest of the world.
Different conditions depending on cell types, etc. Please contact us for more information.
Standard Conditions for Payment